Background
In addition to molecular genetic and cytogenetic findings, gene expression profiles provide independent prognostic information in AML. Aberrant expression of non-coding RNAs, including lncRNAs, further inform pt prognosis. However, non-coding expression profiles and their clinical relevance in AML almost exclusively stem from studies of pts of European ancestry (hereafter referred to as White). Given the inferior survival of African-American (hereafter referred to as Black) AML pts, we hypothesized that aberrant, possibly ancestry-associated lncRNA expression may contribute to survival disparities. Hence, we set out to (1) characterize the lncRNA landscape of African-American ancestry, (2) delineate associations of lncRNAs with clinical and molecular characteristics and (3) assess the prognostic significance of ancestry-associated lncRNAs in AML.
Methods
We used diagnostic samples from 681 adult AML pts (<60y) treated similarly on Alliance frontline protocols (White, n=616; Black, n=65) for RNAseq, mutational profiling and centrally reviewed karyotyping. A negative binomial model was fit to identify lncRNAs differentially expressed between Black and White pts. To determine prognostic relevance of identified lncRNAs, 10-fold cross-validation repeated 1000 times was performed on LASSO penalized Cox proportional hazards model to overall survival (OS). LncRNAs appearing in at least 900 of the 1000 models were retained and coefficient estimates were averaged then applied to form the “lncRNA score.” Ancestry-associated SNPs in regulatory regions of select lncRNAs were identified using the ENCODE registry for cCREs, SNP2TFBS and population allele frequency data from NCBI ALFA.
Results
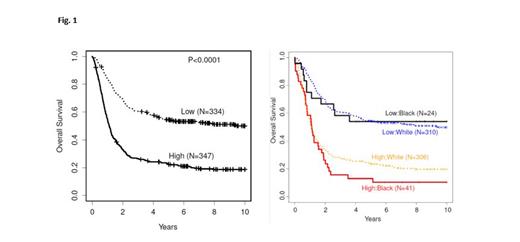
Comparing Black and White AML pts, 135 lncRNAs were upregulated and 55 lncRNAs were downregulated. The differentially expressed lncRNAs were equally distributed throughout the genome and no lncRNA was ancestry-exclusive in its expression, indicating high conservation. lncRNAs with the highest differential expression include several lncRNAs previously implicated in cancer/leukemia, but also thus far unstudied lncRNAs. Using the 190 aberrantly expressed lncRNAs identified from comparison of Black and White pts, we derived a prognostic score (lncRNA score) consisting of 13 lncRNAs. LncRNA High strongly associated with inferior OS in all pts ( Fig. 1). In all pts combined, presence of lncRNA High positively associated with several adverse-risk features, and negatively associated with core-binding factor AML and CEBPA bZIP mutations. Notably, select genetic features differed in their lncRNA score associations based on ancestry, including a relative paucity of cytogenetically normal AML (29% vs 57%), NPM1 (13% vs 40%) and CEBPA bZIP (8% vs 17.5%) mutations in Black pts compared to White pts in the lncRNA Low group. lncRNA score was an independent prognosticator of worse OS in both Black and White pts that refined the prognostication of the European LeukemiaNet 2022 Favorable (P=.004) and Intermediate (P<.001) genetic risk groups. It also provided further prognostic information to the LSC17 score in both low (P<.001) and high (P<.001) LSC17 groups. Examination of the genomic context of regulatory regions within the 13 lncRNAs revealed multiple ancestry-associated SNPs that putatively altered transcription factor / chromatin regulator binding sites in cis-regulatory regions, including rs58515841 (MAF White vs Black, 24% vs 9%, CTCF binding site within ENSG00000284052) and rs1400262 (MAF, 62% vs 10%, REST binding site upstream of NCK1-DT).
Conclusions
We show an ancestry-related landscape of lncRNAs in AML, including an ancestry-associated expression profile of lncRNAs and evidence of ancestry-associated SNPs as expression modulators. A prognosis-associated lncRNA score based on differentially expressed lncRNAs in Black versus White AML pts carries prognostic significance irrespective of ancestry and refines both mutation/cytogenetically-based (ELN 2022) and gene-expression-based (LSC17) prognostic algorithms. Our findings highlight the importance of ancestry-diverse populations in the establishment of prognostic scoring systems to provide ancestry-connected survival prediction.
Support: U10CA180821, U10CA180882, U24CA196171; https://acknowledgments.alliancefound.org.
Clinicaltrials.gov Ids: NCT00048958, NCT00899223, NCT00900224
Disclosures
Walker:Karyopharm Therapeutics Inc.: Consultancy, Current Employment, Current equity holder in publicly-traded company. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Larkin:Debiopharm international: Research Funding; Gilead: Honoraria; Astellas Pharma: Consultancy. Blachly:Epigenetic classification of leukemia: Patents & Royalties: PCT conversion filed; Leukemia Diagnostic Device: Patents & Royalties: Being prosecuted; AbbVie: Consultancy; AstraZeneca: Consultancy; Astellas: Consultancy. Stone:AvenCell: Consultancy; BerGenBio: Consultancy; Cellularity: Consultancy; Takeda: Other: DSMB; Ligand Pharma: Consultancy; GSK: Consultancy; Amgen: Consultancy; Lava Therapeutics: Consultancy; Hermavant: Consultancy; Kura One: Consultancy; Jazz: Consultancy; Rigel: Consultancy; Syntrix: Other: DSMB; Epizyme: Other: DSMB; Aptevo: Other: DSMB; CTI Biopharma: Consultancy; Abbvie: Consultancy. Uy:Jazz: Other: Advisory Board. Stock:Kite: Consultancy; Newave: Honoraria; Servier: Other: Data Safety Monitoring Board/Advisory Board; Kura: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria; Glaxo Smith Kline: Consultancy; Amgen: Honoraria. Woyach:Newave: Consultancy; Loxo: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy; Schrodinger: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Byrd:AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES; American Cancer: Membership on an entity's Board of Directors or advisory committees; Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees. Eisfeld:Karyopharm Therapeutics: Other: spouse employment; Astra Zeneca: Honoraria, Other: CEI Advisory Board; OncLive: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal